Clinical Reasoning: A 22-year-old man with diplopia
Chelsea Meyer, DO, Don Raphael P. Wynn, MD, Stefan M. Pulst, MD, DrMed Ricky Chen, MD, Kathleen Digre, MD Correspondence to Dr. Meyer: chelsea.meyer@hsc.utah.edu
Neurology October 11, 2016; 87 (15)
RESIDENT & FELLOW SECTION Section Editor John J. Millichap, MD
SECTION 1
A 22-year-old previously healthy man presented to an ophthalmology clinic with binocular horizontal diplopia. He had recently traveled to the main island of Hawaii. About 2 weeks after returning home, he developed a severe headache with associated fever, emesis, photophobia, phonophobia, and neck stiffness. He also reported a sensation of pressure in his left eye and both ears but denied any pulsatile tinnitus or transient vision loss. Over the next 2 weeks, his headaches worsened, causing him to wake up frequently in the night. He then developed horizontal diplopia that was worse at a distance and was referred to the neuro-ophthalmology clinic. Examination. The patient had some limitation in neck flexion with associated pain. His visual acuity was 20/20 on the right and 20/25 on the left. Pupillary examination showed no relative afferent defect and visual fields were full. He was found to have bilateral abducens palsy with an esotropia of 30 D and a left hypertropia of 2 D with notable mild right head tilt. The hypertropia was thought to be a partial left 4th nerve palsy with a compensatory right head tilt, although a full Parks-Bielschowsky 3-step test was not preformed to confirm this. He had moderate to severe papilledema on funduscopic examination (figure, A). The remainder of his neurologic and ophthalmologic examination was within normal limits.
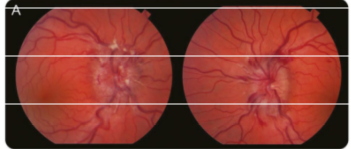
Figure (A) Funduscopic photographs of the optic disc of the left eye show papilledema with blurring of the disc margin, optic nerve hyperemia, and peripapillary hemorrhages.
Questions for consideration:
1. Given his papilledema and bilateral abducens nerve palsy, where would you localize this?
2. What is the differential diagnosis for bilateral abducens nerve palsy?
SECTION 2
The presence of papilledema along with bilateral abducens nerve palsy can occur in the setting of increased intracranial pressure due to a number of etiologies, including a space-occupying lesion (e.g., hemorrhage or tumor), hydrocephalus, cerebral venous sinus thrombosis, idiopathic intracranial hypertension, or meningitis. Abducens nerve palsies, either unilateral or bilateral, can be a false localizing sign; that is, dysfunction of the abducens nerve due to a lesion that is different from the expected anatomical location.1 Theoretically, the long intracranial course of the abducens nerve makes it especially vulnerable to changes in intracranial pressure compared to other cranial nerves. Other etiologies to consider when presented with a bilateral abducens palsy include a pontine tumor resulting in direct compression, clivus tumors, cavernous sinus lesions, Wernicke encephalopathy, myasthenia gravis, Miller Fisher syndrome, or a pseudo-sixth nerve palsy via an upper brainstem infarct resulting in tonic adduction of both eyes.2 Given the patient’s history of headache, any one of the above etiologies should be considered. However, the presence of fever and neck stiffness makes meningitis the more likely etiology at this time.
Question for consideration:
1. What diagnostic evaluation should be performed in this patient?
SECTION 3
The patient’s presentation (headache, fever, diplopia coupled with papilledema, and bilateral 6th nerve palsy) is most concerning for an infectious process, specifically meningitis complicated by dysfunctional CSF circulation resulting in elevated intracranial pressure. For any patient presenting with signs/symptoms concerning for increased intracranial pressure, imaging should be obtained prior to lumbar puncture to rule out a lesion with significant mass effect. In this case, MRI of the brain with and without contrast demonstrated enhancing nodular lesions in the left midbrain and bilateral cortices as well as enhancement along the bilateral optic nerves but no mass lesions (figure, B and C).
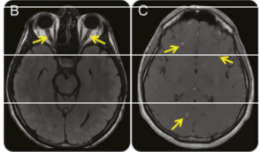
Figures (B) Axial fluid-attenuated inversion recovery image shows hyperintense signal in the distal right and left optic nerves. (C) Axial postcontrast T1-weighted imaging after gadolinium shows enhancing cortical based nodular foci.
Vessel imaging was obtained, including magnetic resonance venography as well as CT angiography of the head and neck, which were all unremarkable (not shown). A CT chest without contrast demonstrated multiple peripheral nodules in the lungs (figure, D).
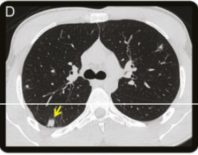
Figure (D) Axial chest CT image shows one of many peripheral lung nodules with mixed attenuation but no associated nodule cavitation.
Following imaging, the patient underwent a lumbar puncture, which revealed an opening pressure of 33 mm CSF with 204 leukocytes/mL (45% lymphocytes, 11% monocytes, and 40% eosinophils), 0 erythrocytes/mL, 151 mg/dL protein, and 36 mg/dL glucose. This profile is indicative of an eosinophilic meningitis, which is defined as an elevated leukocyte count with a cell differential demonstrating greater than 10% eosinophils or simply 10 eosinophils/mL.3
Questions for consideration:
1. What is the differential diagnosis for eosinophilic meningitis?
2. What additional testing would you want to perform?
SECTION 4
The differential diagnosis for eosinophilic meningitis can be divided broadly into infectious and noninfectious etiologies (table). An important noninfectious cause to consider is a medication reaction. Ciprofloxacin, ibuprofen, vancomycin, and gentamicin have all been implicated as potential causes for eosinophilic meningitis.4 Our patient was exposed to ibuprofen; however, his CSF profile demonstrating decreased CSF glucose and elevated protein would be inconsistent with a medication reaction. Malignancies, specifically eosinophilic leukemia and Hodgkin and non-Hodgkin lymphoma, can also cause an eosinophilic meningitis.5 Useful studies in this setting would include a blood smear, peripheral eosinophil count, CSF cytology, and CSF flow cytometry. Other noninfectious etiologies include hypereosinophilic syndrome, neurosarcoidosis, neuromyelitis optica, and ventriculoperitoneal shunts secondary to allergic reactions, which are less likely given the patient’s history and imaging findings.5,6 Infectious etiologies to consider include parasites, fungi, and less commonly, bacteria. The most common parasites responsible for eosinophilic meningitis include Angiostrongylus cantonensis, Gnathostoma spinigerum, and Baylisascaris procyonis, and all 3 can have associated ocular involvement.3 Other parasitic causes of eosinophilic meningitis include neurocysticercosis, cerebral paragonimiasis, neurotrichinosis, cerebral schistosomiasis, and toxocariasis.3 The most common fungal cause of eosinophilic meningitis is Coccidioides meningitis, while potential bacterial causes include syphilis, tuberculosis, and Rickettsia rickettsii.
Questions for consideration:
1. What is the most likely diagnosis?
2. What treatment would you consider?
Table Etiologies of eosinophilic meningitis
Infectious causes of eosinophilic meningitis
Parasites
Angiostrongylus cantonensis
Gnathostoma spinigerum
Baylisascaris procyonis
Taenia solium (Neurocysticercosis)
Paragonimus westermani
Trichinella spiralis (Neurotrichinosis)
Schistosoma haematobium or mansoni
Toxocaria cati or canis (Toxocariasis)
Bacteria
Treponema pallidum (syphilis)
Rickettsia rickettsii
Mycobacterium tuberculosis
Fungi
Coccidioides meningitis
Cryptococcus neoformans or gattii
Noninfectious causes of eosinophilic meningitis
Medications
Ciprofloxacin, ibuprofen, vancomycin, and gentamicin
Malignancies
Eosinophilic leukemia
Hodgkin and non-Hodgkin lymphoma
Autoimmune disorders
Hypereosinophilic syndrome
Neurosarcodosis
Neuromyelitis optica
External devices
Ventriculoperitoneal shunts
SECTION 5
The patient’s presentation and recent travel to Hawaii were most concerning for an infectious etiology, particularly a parasite. He was evaluated for noninfectious causes as well with a complete blood count showing a mild peripheral eosinophilia of 17.5% (normal 0%–6%) but his peripheral smear and CSF cytology/flow cytometry were unremarkable for malignancy or abnormal cells. Of the potential infectious causes, his CSF was tested for bacterial causes like syphilis, Rickettsia, and Bartonella but had negative antibodies. He was tested for fungal etiologies, such as Cryptococcus and Coccidioides (which was a particular concern given the pulmonary nodules), but had negative antibodies for both fungi in his spinal fluid as well as a negative fungal cultures. His diagnosis was eventually made after 2 separate CSF samples were sent to the Centers for Disease Control and Prevention (CDC) for parasitic evaluation—both samples tested positive for A cantonensis in real-time PCR.
DISCUSSION
Prior to confirmatory testing of A cantonensis, the patient’s treatment was purely supportive and included serial lumbar punctures and acetazolamide (a carbonic anhydrase inhibitor) for management of elevated intracranial pressure. Once confirmatory testing came back, and Coccidioides infection was no longer a concern, he was started on a 14-day course of oral prednisone. Overall he had significant improvement in his headache and diplopia with resolution of his abducens palsy on follow-up examination. A cantonensis, also known as the rat lung worm, is a nematode that lives in the pulmonary arteries and alveoli of rats. Its larva can be ingested by mollusks, particularly snails, and then transferred to humans via direct ingestion of the uncooked or undercooked mollusk or ingestion of fresh produce with the mollusk’s slime or larvae.7 It is typically found in Southeast Asia, with the greatest prevalence of disease in Taiwan, but there are reported cases originating in the main island of Hawaii.7 The incubation period can range from days to several months.8 Clinical manifestations are typically headache, neck stiffness, paresthesias, increased intracranial pressure, nausea, and cranial nerve deficits.8 Spinal fluid and serologic analyses typically show an eosinophilic predominant pleocytosis along with a mild to moderate peripheral eosinophilia. Patients may have findings on brain MRI of oval enhancing nodules, meningeal hyperintensities, and nerve root enhancement.9 There have also been reports of pulmonary nodules in the periphery of the lungs, similar to our patient’s CT chest (figure, D).9 Diagnosis of Angiostrongylus meningitis can be challenging because there are no commercially available tests, and the diagnosis is typically made on the clinical history, supportive laboratory/imaging studies, and elimination of other possible causes. In this particular case, CSF was sent to the CDC for confirmatory testing. Angiostrongylus meningitis typically has a self-limiting course but can be lifethreatening secondary to elevated intracranial pressure and require serial lumbar punctures. Treatments with oral corticosteroids have been shown to be useful in treating the headaches along with serial lumbar punctures, but antihelminthics have shown no clear benefit.10
AUTHOR CONTRIBUTIONS
Chelsea Meyer: study concept and design, acquisition of data. DonRaphael P. Wynn: study concept and design, acquisition of data. Stefan Pulst: acquisition of data, analysis and interpretation of data, critical revision of manuscript for intellectual content. Ricky Chen: study concept and design, acquisition of data. Kathleen Digre: acquisition of data, analysis and interpretation of data, critical revision of manuscript for intellectual content.
STUDY FUNDING No targeted funding reported.
DISCLOSURE The authors report no disclosures relevant to the manuscript.
REFERENCES
1. Larner AJ. False localizing signs. J Neurol Neurosurg Psychiatry 2003;74:415–418.
2. Pullicino P, Lincoff N, Truax BT. Abnormal vergence with upper brainstem infarcts: pseudoabducens palsy. Neurology 2000;55:353–358.
3. Lo Re V III, Gluckman SJ. Eosinophilic meningitis. Am J Med 2003;114:217–223.
4. Asperilla MO, Smego RA Jr. Eosinophilic meningitis associated with ciprofloxacin. Am J Med 1989;87: 589–590.
5. Weller PF. Eosinophilic meningitis. Am J Med 1993;95: 250–253.
6. Jarius S, Paul F, Franciotta D, et al. Cerebrospinal fluid findings in aquaporin-4 antibody positive neuromyelitis optica: results from 211 lumbar punctures. J Neurol Sci 2011;306:82–90.
7. Barratt J, Chan D, Sandaradura I, et al. Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology 2016;143:1087–1118.
8. Wang Q, Wu Z, Wei J, et al. Human Angiostrongylus cantonensis: an update. Eur J Clin Microbiol Infect Dis 2012;31:389–395.
9. Jin E, Ma D, Liang Y, Ji A, Gan S. MRI findings of eosinophilic myelomeningoencephalitis due to Angiostrongylus cantonensis. Clin Radiol 2005;60:242–250.
10. Chotmongkol V, Kittimongkolma S, Niwattayakul K, Intapan PM, Thavornpitak Y. Comparison of prednisolone plus albendazole with prednisolone alone for treatment of patients with eosinophilic meningitis. Am J Trop Med Hyg 2009;81:443–445.