Khaled Moussawi, Anoopum Gupta and Haatem Reda
Neurology October 11, 2016; 87 (15)
Correspondence to Dr. Moussawi: khaled.moussawi@nih.gov
RESIDENT AND FELLOW SECTION
Section Editor John J. Millichap, MD
From Harvard Medical School (K.M., A.G., H.R.), Boston; Department of Neurology (K.M., A.G., H.R.), Massachusetts General Hospital, Boston; and Department of Neurology (K.M., A.G.), Brigham and Women’s Hospital, Boston, MA. Dr. Moussawi is currently with the National Institute on Drug Abuse; and the Department of Psychiatry, Johns Hopkins Medicine, Baltimore, MD.
SECTION 1
A 20-year-old man presented to the emergency department with 1 week of headaches and double vision following 2 days of fever (1028 F), nausea, and vomiting. His headache was progressively worsening, throbbing behind his right eye, nonpositional, and associated with photophobia, blurry vision, and pain with eye movement. Occasionally, it was severe enough to wake him up from sleep. Horizontal double vision ensued soon after the headache. His diplopia was worse looking at a distance, improved on leftward gaze, worsened on rightward gaze, and resolved with closing one eye. He denied neck stiffness, focal weakness, numbness, or other neurologic symptoms. He denied recent rashes, infections, or tick bites. He lives on a farm in central Brazil. He arrived in Massachusetts 2 months before his presentation to visit family members. He had no significant medical history and took no medications. He did not smoke or use drugs.
Question for consideration:
1. What is the localization of his presenting symptom?
SECTION 2
Binocular horizontal diplopia that is worse with distance and on rightward gaze is most suggestive of right abducens nerve (cranial nerve [CN] VI) palsy. However, without further information, the differential diagnosis remains broad and encompasses pathologies anywhere between the eye itself and the brainstem. These include but are not limited to eye muscle disease (myositis), orbital lesions (tumors, infections), thyroid disease, neuromuscular junction disorders (myasthenia gravis, botulism), orbital apex infiltrating pathology (tumors, inflammation), cavernous sinus disease (infection, inflammation, thrombosis), clival tumor (chordoma), cerebellopontine angle tumor, ischemic nerve injury, brainstem disease (glioma, demyelination, ischemia), leptomeningeal disease, infectious cranial neuropathy (Lyme, tuberculosis, viral), and increased intracranial pressure (ICP).
Headaches have poor localizing value in general. However, when associated with fever (85% sensitivity for meningitis), nausea, and photophobia, one must consider and rule out meningitis even in the absence of neck stiffness (28% sensitivity for meningitis) or altered mental status (67% sensitivity).e1
On examination, his vital signs and mental status were normal. His neck was supple. No skin rashes were seen. His pupils were normal and visual acuity was 20/20 in each eye. External examination of the eyes and orbits was normal. The examination of the extraocular movements showed a right eye abduction deficit without nystagmus. His visual fields were full. Fundoscopic examination revealed bilateral optic nerve head swelling. Otherwise, his neurologic examination was unremarkable.
Questions for consideration:
1. How would the examination narrow your localization?
2. What would you do next to evaluate the patient?
SECTION 3
His overall clinical presentation of progressive headaches, diplopia, bilateral papilledema, and right CN VI palsy is most consistent with elevated ICP. With a prodrome of fever, nausea, and vomiting, CNS infection needs to be ruled out urgently.
Noncontrast head CT scan was normal. This was followed promptly by a lumbar puncture (LP). The opening pressure was 40 cm H2O. CSF analysis revealed normal protein (37 mg/dL), normal glucose (50 mg/dL), elevated nucleated cells (14/mm3, 83% lymphocytes), few red blood cells (127/mm3 ), normal immunoglobulin G (IgG) index (0.54), and no xanthochromia. The headache improved immediately after the LP. Complete blood count, comprehensive metabolic panel, erythrocyte sedimentation rate, and C-reactive protein were normal. MRI of the brain with contrast demonstrated optic nerve sheath expansion and flattening of the posterior aspects of the globes. No abnormal meningeal or parenchymal enhancement was appreciated (figure e-1 at Neurology.org).
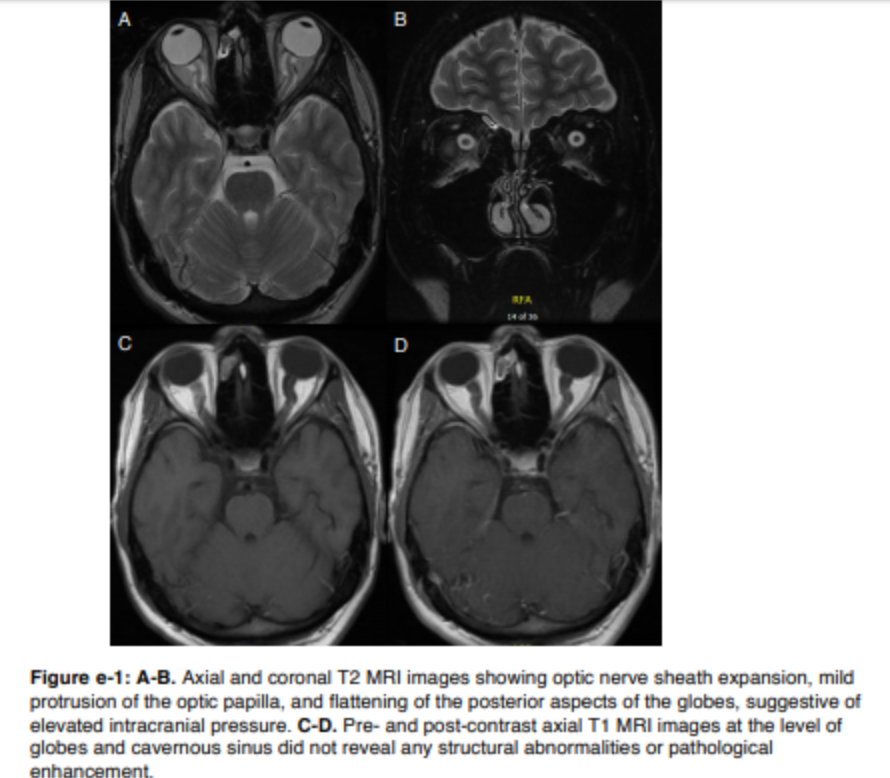
Question for consideration:
1. How would you manage the patient next?
SECTION 4
He was started empirically on IV acyclovir until viral meningitis could be ruled out. For his symptomatic acute intracranial hypertension, he was started on acetazolamidee2 and serial large-volume LPs were performed. A second LP on day 2 was done after recurrence of his headache. Opening pressure was again 40 cm H2O, protein and glucose remained normal, while the nucleated cell count increased to 26/mm3 (94% lymphocytes). Both his headache and right eye abduction improved after the second LP. Similar improvement was noted after a third LP on day 4 (opening pressure 26 cm H2O).
A neuro-ophthalmologic evaluation showed normal visual fields. Visual field testing is very sensitive in assessing damage to the optic nerves from elevated ICP,1 which helps guide further clinical management. For example, if the blind spot were to enlarge and the visual fields become restricted, one ought to consider more invasive interventions such as optic nerve sheath fenestration and/or CSF shunting to avoid permanent optic nerve damage and vision loss.2
However, the most important intervention in similar cases remains to identify and treat the primary etiology of acute intracranial hypertension.
Question for consideration:
1. What is the differential diagnosis for his clinical presentation?
SECTION 5
This patient had a constellation of transient fever, nausea, headache, intracranial hypertension, and CSF lymphocytic pleocytosis, in the absence of clear focal findings on brain MRI. The differential diagnosis includes infectious meningitis, venous sinus thrombosis, carcinomatous meningitis, inflammatory granulomatous meningitis, low-grade glioma, lymphoma, and idiopathic intracranial hypertension.
Our suspicion was highest for infectious meningitis. The infectious differential was especially broad given his history of exposure on a farm in Brazil, but we were most concerned about certain bacterial infections (syphilis, tuberculosis, brucellosis, mycoplasma, Lyme, leptospirosis), fungi (cryptococcosis, coccidiomycosis), parasites (cysticercosis, schistosomiasis), and viruses (herpes simplex virus, HIV, enterovirus, Eastern equine encephalitis, West Nile virus).
While venous sinus thrombosis can be associated with lymphocytic pleocytosis,e3 fever is not usually a presenting symptom. Magnetic resonance venography was performed and was negative. The lack of contrast enhancement on MRI is atypical for neurosarcoidosis or carcinomatous meningitis. Serum and CSF angiotensin-converting enzyme levels were normal and there were no signs of pulmonary sarcoid on chest x-ray. CSF flow cytometry and cytology were also negative. Idiopathic intracranial hypertension is not expected to present with fever or lymphocytic pleocytosis.
There were no HIV or treponemal antibodies detected in the serum, and a purified protein derivative was negative. His CSF bacterial, mycobacterial, and fungal cultures were negative. In addition, CSF VDRL (venereal disease research laboratory), herpes simplex virus and enterovirus PCR, cryptococcal antigen, and serologic tests for cysticercosis, coccidiomycosis, leptospirosis, Eastern equine encephalitis, West Nile virus, and mycoplasma were all negative.
However, he had a positive Schistosoma IgG, which was thought to be from an old infection given his social demographics. Also, Brucella IgM and IgG were positive (titer ,1:80). Repeat testing in 4 weeks showed positive IgM (,1:80) and negative IgG, hence not meeting Centers for Disease Control and Prevention (CDC) criteria for brucellosis.e4
Of note, his Lyme IgM was positive but IgG was negative, which was confirmed by immunoblot (3 bands). The CSF Lyme antibody index did not show intrathecal production of antibodies. Similar Lyme serology was observed on follow-up a month later.
DISCUSSION
This case illustrates an unusual presentation of acute Lyme meningitis, namely, acute intracranial hypertension in the absence of classic meningitic signs such as neck stiffness and altered mental status. The CN VI palsy was most likely a result of elevated ICP rather than infectious mononeuropathy since it improved with serial LPs.
Neurologic manifestations of acute Lyme disease (neuroborreliosis) occur in 12% of patients with Lyme disease.3 These occur 2 to 18 weeks after infection and can be the only manifestation of the disease. The most common presentation of neuroborreliosis in the United States is facial palsy (9% of patients), followed by radiculoneuropathy (4%) and meningitis (1%).e5
Diagnosis of neuroborreliosis requires a 2-tier serologic test and a CSF antibody index to demonstrate intrathecal production of Lyme antibody. The CDC recommends a screening test (usually ELISA). When positive, it is followed by an immunoblot for confirmation ($5 bands for IgG and $2 bands for IgM).e6
Our patient’s blood serology results meet the CDC criteria for acute Lyme infection. Seroconversion from IgM to IgG can take up to 2 months,4 which could explain the positive IgM and negative IgG on repeat testing 1 month after initial presentation. While the definitive diagnosis of Lyme meningitis requires demonstration of intrathecal production of Lyme antibodies, in early neuroborreliosis, the CSF antibody index can be negative in 20% to 30% of patients in the first 6 weeks after symptom onset.5,6
This is the third reported case of acute intracranial hypertension from neuroborreliosis in adults.7,8 However, similar cases have been reported in the pediatric population.e72e12 In these reports, the patients presented with elevated ICP that improved after treatment of Lyme and were described as pseudotumor cerebri from Lyme disease, despite abnormal CSF studies in most reported cases. We disagree with the diagnosis of pseudotumor cerebri, which requires normal CSF composition and usually reflects a specific clinical entity with poorly understood pathophysiology.2 The resolution of all symptoms and signs of elevated ICP after treatment of Lyme favors the diagnosis of secondary intracranial hypertension from meningitis. The specific pathogenesis of elevated ICP in Lyme meningitis is not clear. Nevertheless, we believe this is attributable to the inflammatory response in meningitis, which impairs CSF reabsorption in arachnoid villi. It is well described that purulent meningitis results in deposition of macromolecular proteins such as fibrinogen on the endothelium of arachnoid villi. This impairs bulk flow reabsorption of CSF into the venous circulation, and hence increases ICP.9,e3
Our patient was treated with IV ceftriaxone (2 g/d) for 4 weeks.10 On follow-up 1 month later, he was completely asymptomatic and his examination was normal.
AUTHOR CONTRIBUTIONS
Khaled Moussawi: conceived, wrote, and revised the manuscript. Anoopum Gupta: conceived and revised the manuscript. Haatem Reda: conceived and revised the manuscript. STUDY
FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript.
REFERENCES
1. Wall M. The importance of visual field testing in idiopathic intracranial hypertension. Continuum 2014;20: 1067–1074.
2. Wakerley B, Tan M, Ting E. Idiopathic intracranial hypertension. Cephalalgia 2015;35:248–261.
3. Koedel U, Fingerle V, Pfister HW. Lyme neuroborreliosis: epidemiology, diagnosis and management. Nat Rev Neurol 2015;11:446–456.
4. Aberer E, Schwantzer G. Course of antibody response in Lyme borreliosis patients before and after therapy. ISRN Immunol 2012;2012:1–4.
5. Ljøstad U, Skarpaas T, Mygland A. Clinical usefulness of intrathecal antibody testing in acute Lyme neuroborreliosis. Eur J Neurol 2007;14:873–876.
6. Tumani H, Nölker G, Reiber H. Relevance of cerebrospinal fluid variables for early diagnosis of neuroborreliosis. Neurology 1995;45:1663–1670.
7. Nord JA, Karter D. Lyme disease complicated with pseudotumor cerebri. Clin Infect Dis 2003;37:e25–e26.
8. Castaldo JE, Griffith E, Monkowski DH. Pseudotumor cerebri: early manifestation of adult Lyme disease. Am J Med 2008;121:e5–e6.
9. Merritt H, Fremont-Smith F. Acute purulent meningitis. In: Merritt H, editor. The Cerebrospinal Fluid. Philadelphia: WB Saunders; 1938:94–103.
10. Halperin JJ. Nervous system Lyme disease. Handb Clin Neurol 2014;121:1473–1483.
Supplemental references:
e-1 Attia J, Hatala R, Cook DJ, Wong JG. The rational clinical examination. Does this adult patient have acute meningitis? Jama. 1999 Jul 14;282(2):175–181.
e-2 Rubin RC, Henderson ES, Ommaya AK, Walker MD, Rall DP. The production of cerebrospinal fluid in man and its modification by acetazolamide. JNeurosurg. 1966 Oct;25(4):430–436.
e-3 Fishman RA. Cerebrospinal fluid in diseases of the nervous system. 1992.
e-4 http://www.cdc.gov/brucellosis/clinicians/serology.html
e-5 http://www.cdc.gov/lyme/stats/graphs.html
e-6 http://www.cdc.gov/lyme/diagnosistesting/labtest/twostep/index.html
e-7 Steenhoff AP, Smith MJ, Shah SS, Coffin SE. Neuroborreliosis with progression from pseudotumor cerebri to aseptic meningitis. Pediatr Infect Dis J. 2006 Jan;25(1):91–92.
e-8 Raucher HS, Kaufman DM, Goldfarb J, Jacobson RI, Roseman B, Wolff RR. Pseudotumor cerebri and Lyme disease: A new association. The Journal of Pediatrics. 1985 Dec;107(6):931–933.
e-9 Kan L MD, Sood SK MD, Maytal J MD. Pseudotumor Cerebri in Lyme Disease: A Case Report and Literature Review. Pediatr Neurol. 1998 May;18(5):439–441.
e-10 Härtel C, Schilling S, Neppert B, Tiemer B, Sperner J. Intracranial hypertension in neuroborreliosis. Dev Med Child Neurol. Cambridge University Press; 2002 Sep 1;44(09):641–642.
e-11 Portmann A, Gueudry J, Lebas A, et al. [Isolated intracranial hypertension as the presenting sign of Lyme disease]. J Fr Ophtalmol. 2012 Nov;35(9):720.e1–.e4.
e-12 Steenhoff AP, Smith MJ, Shah SS, Coffin SE. Neuroborreliosis with progression from pseudotumor cerebri to aseptic meningitis. Pediatr Infect Dis J. 2006 Jan;25(1):91–92