Questions:
1. When examining the pupils, what should you record?
2. Does a RAPD cause anisocoria?
3. If a patient is suspected of having optic neuropathy (regardless of the cause) has no RAPD does that rule-out this diagnosis?
4. If a patient has a severe bilateral optic neuropathy will the pupils respond to near stimuli?
5. Which order neuron is involved when the Horner syndrome is caused by a tumor in the apex of a lung?
6. Why do patients with a third-order Horner syndrome usually do not have anhidrosis?
7. What is neurotransmitter is released at the neuromuscular junction of the iris sphincter to result in pupillary constriction?
8. What is neurotransmitter is released at the neuromuscular junction of the iris sphincter to result in pupillary dilation?
9. Why do lesions of the geniculate nucleus, the optic radiations, or the visual cortex not affect pupillary size or pupillary reactivity?
10. What is the course of the parasympathetic fibers for pupillary constriction from the Edinger-Westphal nucleus to the ciliary ganglion?
11. What is the ratio of postganglionic parasympathetic fibers that innervate the ciliary muscle to those that innervate the pupillary sphincter muscle?
Questions with answers:
1. When examining the pupils, what should you record?
● Size (in millimeters)
● Presence of anisocoria
● Response to light (direct and consensual response)
● Presence of a relative afferent pupillary defect (RAPD)
● Dilation in the dark
● Constriction at near
2. Does a RAPD cause anisocoria?
No. A relative afferent pupillary defect will not cause.
3. If a patient is suspected of having optic neuropathy (regardless of the cause) has no RAPD does that rule-out this diagnosis?
No. If a patient with a suspected optic neuropathy (regardless of the cause) has no RAPD, either the patient does not have an optic neuropathy or the optic neuropathy is bilateral.
4. If a patient has a severe bilateral optic neuropathy will the pupils respond to near stimuli?
Yes. Because the afferent pathways serving the light reflex and the near reflex are anatomically distinct, patients with severe optic neuropathies will still have intact, brisk pupillary responses to near stimuli, while their pupils will not, or will only poorly, react to light’
5. Which order neuron is involved when the Horner syndrome is caused by a tumor in the apex of a lung?
Second-order neuron: travels from the sympathetic trunk, through the brachial plexus, and over the lung apex. It then ascends to the superior cervical ganglion (located near the angle of the mandible and the bifurcation of the common carotid artery).
6. Why do patients with a third-order Horner syndrome usually do not have anhidrosis?
The sympathetic fibers responsible for facial sweating and vasodilation branch off at the superior cervical ganglion from the remainder of the oculosympathetic pathway (explaining why patients with a third-order Horner syndrome usually do not have anhidrosis [loss of sweating]).
7. What is neurotransmitter is released at the neuromuscular junction of the iris sphincter to result in pupillary constriction?
Acetylcholine
8. What is neurotransmitter is released at the neuromuscular junction of the iris sphincter to result in pupillary dilation?
Norepinephrine
9. Why do lesions of the geniculate nucleus, the optic radiations, or the visual cortex not affect pupillary size or pupillary reactivity?
Afferent pupillary fibers leave the optic tract before the lateral geniculate nucleus via the brachium of the superior colliculus to reach the pretectal nuclei (explaining why lesions of the geniculate nucleus, the optic radiations, or the visual cortex do not affect pupillary size or pupillary reactivity, and why lesions of the brachium of the superior colliculus can cause a relative afferent pupillary defect without visual loss).
10. What is the course of the parasympathetic fibers for pupillary constriction from the Edinger-Westphal nucleus to the ciliary ganglion?
Parasympathetic fibers for pupillary constriction leave the Edinger–Westphal nucleus and travel along the ipsilateral third cranial nerve to the ipsilateral ciliary ganglion within the orbit.
11. What is the ratio of postganglionic parasympathetic fibers that innervate the ciliary muscle to those that innervate the pupillary sphincter muscle?
30 to 1
Explanation: “12 The Pupil
Pupillary function is an important objective clinical sign in patients with visual loss and neurologic disease.
The mnemonic PERRLA (pupils equal, round, reactive to light, and accommodation)reminds us of the four questions we should ask in evaluating the pupils:
● Are the pupils equal in size?
● Are the pupils round or irregularly shaped?
● Do the pupils react to a light stimulus?
● If not, do they react to a near target?
The normal pupil varies in size, depending on the ambient illumination. Pupils are usually symmetrical in size, although physiologic anisocoria (the difference in size between the two pupils) of 0.4mm or greater is seen in about 20% of individuals.
12.1 Examining the Pupils
Pupils should be tested in the dark with a bright light and with the patient fixating at distance.
When examining the pupils, you should record the following:
● Size (in millimeters)
● Presence of anisocoria
● Response to light (direct and consensual response)
● Presence of a relative afferent pupillary defect (RAPD)
● Dilation in the dark
● Constriction at near
When pupillary reactions are abnormal, slit lamp examination of the anterior segment and the iris may demonstrate abnormalities that may affect pupillary size and shape, such as synechiae, uveitis, iris tear, segmental contraction of the iris, iris tumor, and lens subluxation (▶Table 12.1).

Pearls
Shining a light in one eye of a normal subject causes both pupils to constrict equally. The pupillary response in the illuminated eye is called the direct response. The pupillary response in the eye that is not being illuminated is called the consensual response.
12.2 Clinical Anatomy and Physiology of the Pupils
Light directed into either eye normally produces bilateral pupillary constriction.
Each pupil receives both sympathetic (dilator muscle: active dilation) and parasympathetic (sphincter muscle: active constriction) innervation. The size of the pupils at anyone moment is determined by the balance of the parasympathetic tone of the iris sphincter and the sympathetic tone of the iris dilator. This balance is in constant flux, so that pupil sizes change symmetrically from moment to moment.
Hippus is the normal rhythmic pupillary oscillation commonly seen when light stimulates either eye. Both pupils oscillate in synchrony, and the amplitude and frequency vary (▶Table 12.2).
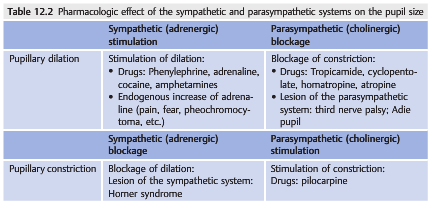
Changes in the pupil innervation will produce dilation or constriction of the pupil. Additionally, local problems such as lesions of the iris (tumor, iris synechiae on the lens, iris tears from trauma, and postsurgical pupil) may change the shape or alter the reactivity of the pupil.
Pupillary size results from the balance of actions of two opposing muscle groups of the iris: the dilator of the iris (responsible for dilation) and the sphincter pupillae (responsible for constriction). The size changes are reflex mechanisms in response to the amount of ambient light. These changes vary based on the patient’s age, emotional state (adrenergic tone), state of arousal, and intraocular pressure.
12.2.1 Pupillary Light Reflex Pathway
Pupillary constriction to light is mediated via parasympathetic (cholinergic) nerve fibers that travel along the third cranial nerve. When light is shone into one eye, both pupils constrict symmetrically (direct and consensual response to light). Light information from retinal ganglion cells travels through the optic nerves, chiasm (where the nasal fibers decussate), and optic tracts to reach the pretectal nuclei of the dorsal midbrain (▶Fig. 12.3).
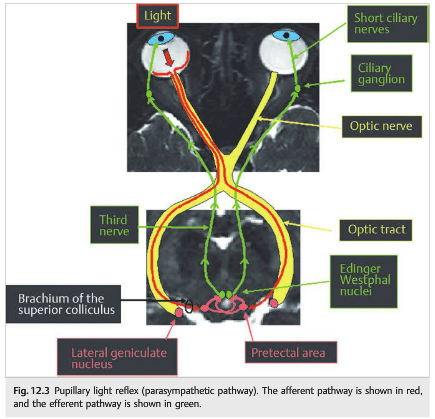
Afferent pupillary fibers leave the optic tract before the lateral geniculate nucleus via the brachium of the superior colliculus to reach the pretectal nuclei (explaining why lesions of the geniculate nucleus, the optic radiations, or the visual cortex do not affect pupillary size or pupillary reactivity, and why lesions of the brachium of the superior colliculus can cause a relative afferent pupillary defect without visual loss).
Both pretectal nuclei receive input from both eyes, and each sends axons to both Edinger–Westphal nuclei (connections are bilateral but predominantly from the contralateral nucleus). Parasympathetic fibers for pupillary constriction leave the Edinger–Westphal nucleus and travel along the ipsilateral third cranial nerve to the ipsilateral ciliary ganglion within the orbit.
The postganglionic parasympathetic fibers innervate the ciliary muscle (for lens accommodation) and the pupillary sphincter muscle (for pupil constriction) in a proportion of 30:1. (This is important to understand the pathophysiology of the tonic[Adie] pupil.) Acetylcholine is released at the neuromuscular junction of the iris sphincter to result in pupillary constriction.
12.2.2 Relative Afferent Pupillary Defect
When light is directed into either eye, both pupils react equally. The brighter the light source the greater the degree of bilateral pupillary constriction. Therefore, the amount of pupillary constriction from the same light source directed to either eye should be identical.
The RAPD, where stimulation of one eye causes both eyes to constrict less than stimulation of the other eye, is a very important objective sign of optic nerve disease. It can easily be detected at bedside, and it can also be quantified (see Chapter 1).
In unilateral optic nerve or retinal ganglion cell dysfunction, the light signal received by the brainstem efferent centers is relatively less when the same light source is presented to the affected eye. Hence both pupils constrict less when the involved eye is stimulated and more when the normal eye is stimulated (▶Fig. 12.4).
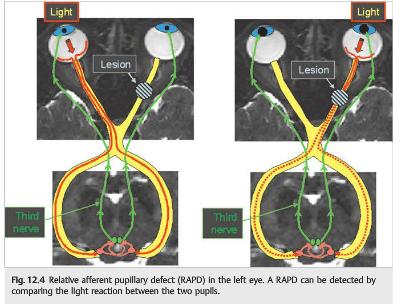
A RAPD ipsilateral to visual loss indicates an optic neuropathy or severe retinal disease (in which case the retina looks abnormal on funduscopic examination). Ocular disease, such as corneal abnormalities, cataracts, and most retinal disorders, do not cause a RAPD.
A unilateral lesion in the pretectal nucleus or in the brachium of the superior colliculus will damage the pupillary fibers coming from the ipsilateral optic tract. This can produce a contralateral RAPD, just like in optic tract lesions, but without any visual loss or visual field defect.
By placing neutral-density filters over the normal eye, the examiner can neutralize the RAPD and quantitate its severity in log units (see Chapter 1). Filters usually range from 0.3 (small RAPD) to 1.8 (dense RAPD) log units.
The causes of RAPD include the following:
● Unilateral or asymmetric optic neuropathy (0.3 to 1.8 log units)
● Severe unilateral retinopathy (0.3–>1.8 log units)
● Maculopathy with visual acuity worse than 20/200 (small RAPD of 0.3 log unit)
● Amblyopia (small RAPD of 0.3 log unit)
● Dense, unilateral cataract: may produce a small contralateral RAPD (the retina behind the dense cataract is dark adapted, and the light shines in all directions, causing excess retinal stimulation on the side of the dense cataract)
● Patching of one eye (or complete ptosis) may produce a transient contralateral RAPD(the patched eye has a dark-adapted retina and is hypersensitive to light; this can produce a contralateral RAPD of up to 1.5 log units for up to 30 minutes after the patch is removed).
● Optic tract lesions produce a contralateral RAPD (RAPD of 0.3–0.6 log unit), which is on the side of the homonymous hemianopia, the side opposite the lesion (see Chapter 3, ▶Fig. 3.22).
● Lesions of the brachium of the superior colliculus or the pretectal nucleus produce contralateral RAPD without visual loss or visual field defect.
Pearls
1. A relative afferent pupillary defect will not cause anisocoria (inequality in size of the pupils).
2. If a patient with a suspected optic neuropathy (regardless of the cause) has no RAPD, either the patient does not have an optic neuropathy, or the optic neuropathy is bilateral.
12.2.3 Pupillary Constriction with Accommodation
Pupillary constriction to a near stimulus is accomplished through the parasympathetic pathways. The near-reflex pathway bypasses the pretectal nuclei in the dorsal midbrain and descends directly to the area of the Edinger–Westphal nuclei from higher cortical centers.
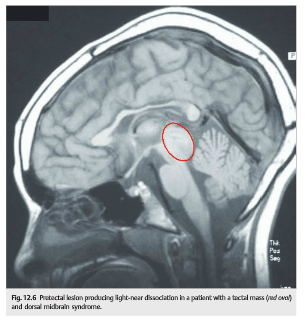
The distinction between the light-reflex and near-reflex pathways forms the basis for some forms of pupillary light-near dissociation (i.e., pupils that do not react to light but react to near stimuli) in which the dorsal midbrain and pretectal nuclei are damaged, but the near-reflex pathways and the Edinger–Westphal nuclei are spared (▶Fig. 12.5 and▶Fig. 12.6)
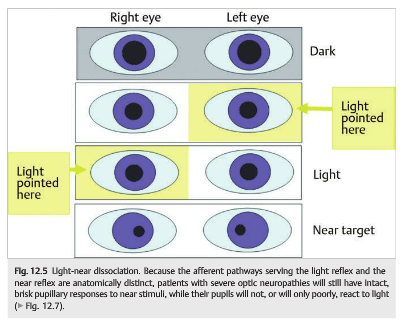
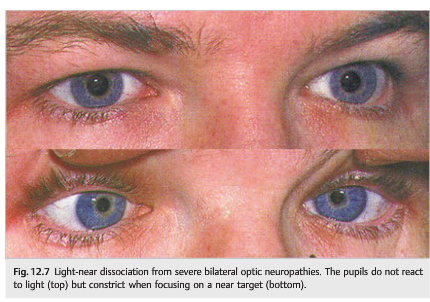
Because the afferent pathways serving the light reflex and the near reflex are anatomically distinct, patients with severe optic neuropathies will still have intact, brisk pupillary responses to near stimuli, while their pupils will not, or will only poorly react to light (▶Fig. 12.7).
12.2.4 Pupillary Dilation
Pupillary dilation is mediated through sympathetic (adrenergic) pathways that originate in the hypothalamus (▶Fig. 12.8).
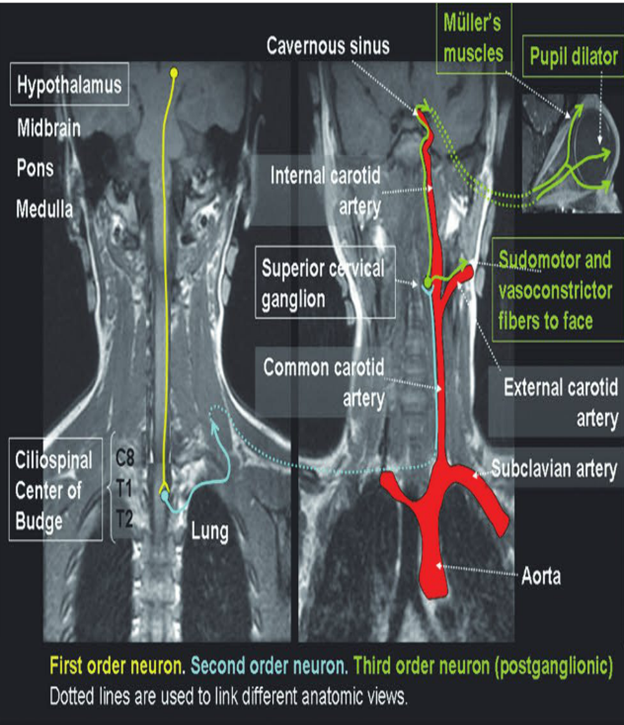
Fig.12.8 Sympathetic pupillary pathways. The three-neuron pathways are represented on two different coronal views of the upper chest, neck, and head.
The sympathetic pathway (oculosympathetic fibers) responsible for pupillary dilation is a three-neuron pathway:
● First order neuron: descends from the hypothalamus to the first synapse, which is located in the cervical spinal cord (levels C8–T2, also called the intermediolateral cell column or the ciliospinal center of Budge). In the midbrain, the sympathetic pathway is located close to the fourth nerve nucleus.
● Second-order neuron: travels from the sympathetic trunk, through the brachial plexus, and over the lung apex. It then ascends to the superior cervical ganglion (located near the angle of the mandible and the bifurcation of the common carotid artery).
● Third-order neuron (distal to the superior cervical ganglion): ascends within the adventitia of the internal carotid artery and through the cavernous sinus (where it is in close relation to the sixth cranial nerve) and joins the ophthalmic (V 1) division of the fifth cranial nerve to get into the orbit.
The oculosympathetic fibers innervate the iris dilator muscle, Müller muscles (small muscles in the upper eyelids responsible for a minor portion of upper lid elevation), and inferior tarsal muscle (equivalent of the Müller muscles in the lower eyelid).
The sympathetic fibers responsible for facial sweating and vasodilation branch off at the superior cervical ganglion from the remainder of the oculosympathetic pathway (explaining why patients with a third-order Horner syndrome usually do not have anhidrosis [loss of sweating]).
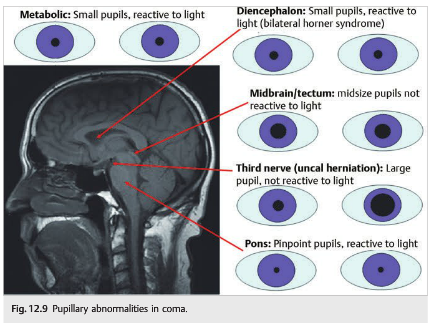
12.3 Pupillary Abnormalities in Coma
The metabolic causes of coma usually cause small reactive pupils (▶Fig. 12.9). Many Toxins and drugs also have effects on the size of the pupils. Finally, pharmacologic mydriasis can inadvertently occur in patients treated with aerosols after extubation.
Reference: 1. Neuro-ophthalmology Illustrated-2nd Edition. Biousse V and Newman NJ. 2012. Theme
These questions are archived at https://neuro-ophthalmology.stanford.edu
Follow https://twitter.com/NeuroOphthQandA to be notified of new neuro-ophthalmology