Question: Which of the following are hyperautofluorescent?
1. Optic Disc Drusen
2. Papilledema
3. Accumulation of lipofuscin in the retinal pigment epithelium
4. Loss of lipofuscin in the retinal pigment epithelium
5. Central serous chorioretinopathy
6. Best disease
 1
1
____________________________________________________
Correct Answers:
1. Optic Disc Drusen
3. Accumulation of lipofuscin in the retinal pigment epithelium
5. Central serous chorioretinopathy
6. Best disease
Explanation: “4.2 Fundus Autofluorescence Imaging
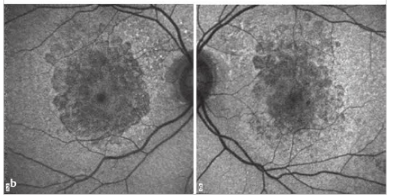
Fundus autofluorescence (FAF) imaging is helpful in diagnosing retinal conditions at an early stage by showing abnormalities that are often invisible to standard fundus photography and ophthalmoscopy (▶Fig. 4.5).
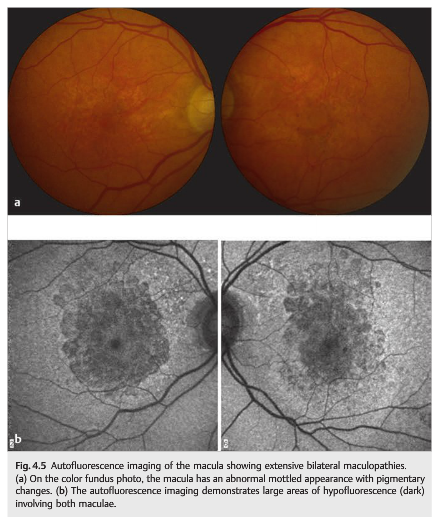
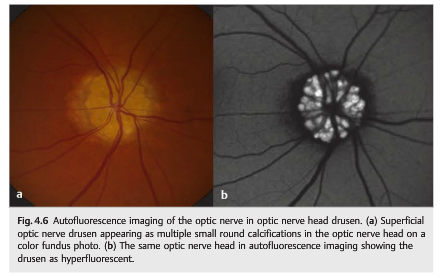
Autofluorescence is caused by the presence of lipofuscin, an aging pigment fluorophore produced by the outer segments of the photoreceptors and stored at the level of the retinal pigment epithelium. Two abnormal states of lipofuscin exist, hyperautofluorescence and hypoautofluorescence (▶Fig. 4.5b); both are associated with various retinal disorders. Optic nerve head drusen are also usually hyperautofluorescent (▶Fig. 4.6).
 ”1
”1
The Nuts and Bolts of Fundus Autofluorescence Imaging. Stuart A et. al. 2012. AAO EyeNet Magazine.
“Unlike other imaging modalities”, said Dr. Kiss, “FAF provides functional information about retinal cells.” According to Dr. Spaide, “it is useful for almost any fundus disorder, including AMD, retinal detachment, inherited dystrophies, central serous chorioretinopathy, vitelliform lesions, and acute zonal occult outer retinopathy (AZOOR). Dr. Kiss noted that FAF is also helpful in screening for medication toxicity, including eye problems related to hydroxychloroquine (Plaquenil).”
AMD “FAF imaging allows functional evaluation of geographic atrophy in dry AMD”, said Dr. Kiss, “making it possible to assess emerging therapies and monitor response to medication as well as progression of the disease.”
“Areas of geographic atrophy are hypofluorescent because there are no photoreceptors and no lipofuscin within the RPE cells,” he said. “However, hyperfluorescence shows up in junctional zones around geographic atrophy where the RPE is working overtime – a foreshadowing of imminent atrophy.”
“Of the studies using autofluorescence as a secondary end point, the most prominent one is AREDS 2,” said Dr. Kiss. “The results may have a strong effect on the application of FAF imaging in the future”, he added,” just as OCT now influences clinicians’ treatment of patients with wet AMD.”
Retinal detachment “Hyperfluorescence in areas immediately adjacent to a retinal detachment can demarcate its extent and help explain visual problems in patients”, said Dr. Kiss. “In a small series using ultra-wide-field FAF imaging, we also showed that these areas can remain abnormal, even after the retina is reattached.”
Dystrophies and degenerations “Retinal dystrophies and degenerations also show abnormal autofluorescence”, said Dr. Kiss. “As with dry AMD, retinal dystrophies such as retinitis pigmentosa demonstrate areas of both hyper- and hypofluorescence, a sign that the retina is burning out.”
“Always test Best disease,” said Dr. Kiss, “which is characterized by macular or submacular accumulation of lipofuscin material and has a sharp, characteristic hyperfluorescent region in the macula.” Dr. Spaide added, “We are familiar with Best disease causing a yellow egg yolk–like lesion in kids and young adults, but with time that will go away. However, FAF imaging shows widespread abnormalities in the fundus and also can find areas of atrophy within the lesions.”
Dr. Sadda finds FAF particularly useful in diagnosing Stargardt disease, in which pisciform lesions are readily apparent.
Central serous chorioretinopathy (CSC). According to Dr. Spaide, the extent of CSC is best seen with autofluorescence, not just in the area of the subretinal fluid but also in other parts of the macula or even the other eye.
“As with diseases that cause a buildup of vitelliform material”, said Dr. Spaide, “CSC accumulates outer segments that have been shed but not yet phagocytized. In central serous chorioretinopathy, it is common to see subretinal accumulation of material,” he said. “Some ophthalmologists will call that ‘subretinal protein’ without giving the material much thought. However, it is highly autofluorescent in the wavelengths used to excite retinoids. Proteins do not efficiently autofluorescence in these wavelengths, so the hypothesis that the material is protein does not fit the available facts.”2
References:
1. Neuro-ophthalmology Illustrated-2nd Edition. Biousse V and Newman NJ. 2012. Theme
2. The Nuts and Bolts of Fundus Autofluorescence Imaging. Stuart A et. al. 2012. AAO EyeNet Magazine.
More than 600 additional neuro-ophthalmology questions are freely available at http://EyeQuiz.com.
Questions prior to September 2016 are archived at http://ophthalmology.stanford.edu/blog/
After that, questions are archived at https://neuro-ophthalmology.stanford.edu
Follow https://twitter.com/NeuroOphthQandA to be notified of new neuro-ophthalmology questions of the week.
Please send feedback, questions and corrections to tcooper@stanford.edu.