 1
1
Question:
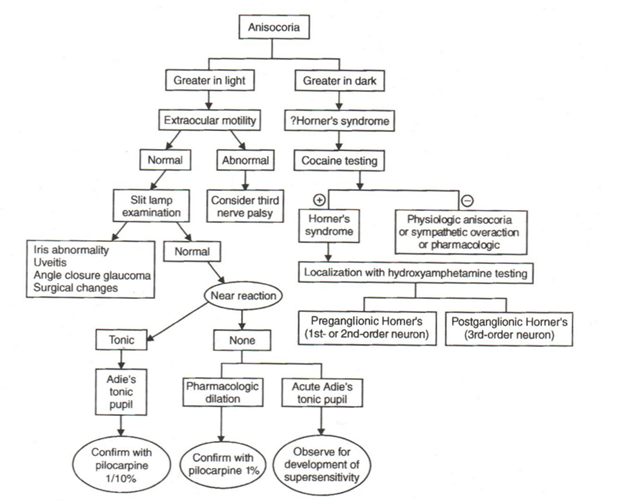
Describe the appropriate steps to take in the emergency department when evaluating anisocoria.
ED Concerns: apical lung lesion, carotid artery dissection, cerebral aneurysm, intracranial mass lesion
ED Evaluation:
Determine if the anisocoria is more pronounced in the light or in the dark by measuring the pupil diameters under these 2 conditions.
A. Anisocoria greater in the dark-small pupil is abnormal
– a. Rule-out Horner syndrom
– Ptosis present on side of smaller pupil
➧ Anisocoria onset recent
⏩ Obtain emergent MRI of Brain/Neck/Superior Chest
➧ Anisocoria onset NOT recent
⏩ Arrange neuro-ophthalmology clinic appointment
B. Anisocoria greater in the light-large pupil is abnormal
– a. Assess extraocular motility
– Normal
➧ Perform slit-Lamp exam to rule-out iris abnormality (surgical or other traumatic defects), uveitis, angle closure glaucoma
⏩ Normal arrange neuro-ophthalmology clinic appointment
⏩ Abnormal treat acute problems and arrange ophthalmology follow-up
– Abnormal – Consider third nerve palsy
➧ Obtain emergent MRI Brain/orbits and MRA Brain
➧ Arrange emergent neurology/neurosurgery consultation
C. Anisocoria of 1 mm. or less and not more pronounced in the light or in the dark = physiologic anisocoria
– a. Arrange neuro-ophthalmology clinic appointment
Explanation: The elimination of recent onset Horner syndrome and 3rd nerve paresis in the ED is essential to ensure that conditions requiring emergent treatment are not missed. If no emergency is present an evaluation in neuro-ophthalmology is appropriate.
It is best to avoid pharmacologic testing in the ED to:
1. avoid confusing other physicians who may subsequently observe the patient to have a changed pupillary examination,
2. permit time for supersensitivity to develop for apraclonidine and 0.1% pilocarpine testing, and
3. pharmacologic assessment of the cause of anisocoria can yield false positive and false negative results.
“Pharmacologic anisocoria can present as mydriasis or miosis following administration of agents that act on the pupillary dilator or sphincter muscles. Anticholinergics such as atropine, homatropine, tropicamide, scopolamine and cyclopentolate lead to mydriasis and cycloplegia by inhibiting parasympathetic M3 receptors of the pupillary sphincter and ciliary muscles. The use of pilocarpine, a non-selective muscarinic receptor agonist in the parasympathetic nervous system, may result in a small and poorly reactive pupil. Sympathomimetics such as adrenaline, and phenylephrine cause mydriasis through their actions at ɑ-1 receptors of the pupillary dilator muscle.”5
Video Horner Syndrome Dilation Lag – Moran Eye Center University of Utah
http://morancore.med.utah.edu/section-05-neuro-ophthalmology/dilation-lag/
Pupillary dilation lag is intermittently present in patients with a stable oculosympathetic defect (Horner syndrome). Crippa SV, Borruat FX, Kawasaki A. Am J Ophthalmol. 2007; 143(4):712-5
PURPOSE: To examine the repeatability of detecting pupillary dilation lag in patients with Horner syndrome.
DESIGN: Retrospective interventional study.
METHODS: Setting: Single referral institution. Patient population: Fifteen patients with unilateral Horner syndrome and 16 subjects with physiologic anisocoria. Intervention procedure: Each subject underwent four pupillometric recordings in darkness. The asymmetry of pupillodilation between the two eyes was calculated as the change in anisocoria between five seconds and 15 seconds in darkness. Pupillary dilation lag was considered present if the asymmetry measured > or =0.4 mm. Main outcome measure: Asymmetry of pupillodilation over four determinations.
RESULTS: All subjects demonstrated fluctuations in the calculated asymmetry of pupillodilation. Eight patients(53%) with Horner syndrome demonstrated dilation lag on the first determination; 13 patients (87%) eventually demonstrated it during four determinations.
CONCLUSIONS: Pupillary dilation lag is intermittently present in most patients with Horner syndrome. Repeated observations improve the detection rate of dilation lag, a confirmatory sign of an oculosympathetic deficit. Its absence does not rule out Horner syndrome.
The cocaine test in normal patients. Friedman JR, Whiting DW, Kosmorsky GS, Burde RM. Am J Ophthalmol. 1984;98:808-81
Of 24 normal patients 20 had less than 0.5 mm of asymmetry in response to two drops of 10% topical cocaine five minutes apart. “The development of anisocoria of more than 0.5 mm is necessary for the diagnosis of Horner’s syndrome to be made using cocaine.” Individual variation between the two normal eyes, “although minimal, does exist in white patients. No such conclusion could be drawn for black patients because of their overall lack of response to the application of topical cocaine routinely used. The cocaine test may not be appropriate for use in black patients to diagnose Horner’s syndrome.
The diagnosis of Horner’s syndrome: use and limitations of the cocaine test. Van der Wiel HL, Van Gijn J. J Neurol Sci. 1986;74:311-316
We prospectively studied the value of the cocaine test in the diagnosis of Horner’s syndrome, by performing the test in 20 control subjects and in 20 patients with a provisional diagnosis of Horner’s syndrome. Photographic testing of the darkness reflex of the pupil was used as an independent criterion of oculosympathetic dysfunction, and confirmed the diagnosis in 12 of the 20 patients. A mydriatic response to cocaine that was at least 1.0 mm less than in the unaffected eye occurred only with Horner’s syndrome (7 patients). On the other hand, if the difference is smaller than 1.0 mm the chance that the patient does not have Horner’s syndrome is only around 60%. There was no relationship between the magnitude of the response to cocaine and the site of the lesion in the sympathetic system.
… if a difference between the two eyes of 1.0 mm or more is considered to be evidence of Horner’s syndrome, the predictive value of an abnormal test result is 100 % (7/7; 95 % confidence interval 59-100%), that of a normal test result 62 % (8/13; 95 % confidence interval 32-86 %).
… In the only two black control subjects (with dark brown irises), the pupils dilated by only 0.3 mm or less, which was confirmed on repeated testing.”
Critical evaluation of the cocaine test in the diagnosis of Horner’s syndrome – Kardon et al, – Arch Ophthalmol. 1990;108:384-387
We evaluated the effectiveness of the cocaine test for diagnosing Horner’s syndrome. The test was administered to 119 patients with a diagnosis of Horner’s syndrome and to 50 normal subjects. We compared the cocaine-induced anisocoria in the two groups by measuring photographs of the pupils. We found the cocaine test to be highly effective in separating normal subjects from patients with Horner’s syndrome. The chances of having Horner’s syndrome increased with the amount of cocaine-induced anisocoria. Through the use of logistic regression analysis, we determined the odds ratio of having Horner’s syndrome compared with not having it for each 0.1-mm increment of anisocoria measured after cocaine administration. A postcocaine anisocoria value of 0.8 mm gave a mean odds ratio of approximately 1050:1 that Horner’s syndrome was present (lower 95% confidence limit ° 37:1). (emphasis added) We found that simply measuring the post cocaine anisocoria provided a better prediction of Horner’s syndrome than taking the trouble to calculate the net change in anisocoria. Odds ratios should help the clinician decide if the result of a cocaine test is indicative of Horner’s syndrome.
Duration of positive urine for cocaine metabolite after ophthalmic administration: implications for testing patients with suspected Horner syndrome using ophthalmic cocaine. Jacobson DM, Berg R, Grinstead GF, Kruse JR. Am J Ophthalmol. 2001 Jun;131(6):742-7.
Patients should be informed that their urine may test positive for cocaine, if assayed according to US federal guidelines and using the protocol employed in this study, up to 2 days after undergoing testing for Horner syndrome.
Hydroxyamphetamine mydriasis in Horner’s syndrome. Cremer SA, Thompson S, Digree KB, et al. Am J Ophthalmol 1990;110:71–6.
Using 1% Hydroxyamphetamine 1 drop to each eye, the eyes wiped, and a second drop administered about 20-40 seconds later, the mean probability that a patient with a 1.5-mm difference in dilation has a postganglionic lesion in the oculosympathetic pathway was 96% while a difference of 1.0-mm had a mean probability of 85.5%. In this study 5 of 30 patients with postganglionic lesions had a difference in dilation of less than 1.0.mm.
When the lesion was judged to be preganglionic or central (nonpostganglionic) the difference in dilation was negative or zero 83% of the time (specificity = 83%).
The sensitivity and specificity of 0.5% apraclonidine in the diagnosis of oculosympathetic paresis. F Koc, S Kavuncu, T Kansu, G Acaroglu, E Firat. Br J Ophthalmol 2005;89:1442–1444.
Aims: To evaluate the sensitivity and specificity of 0.5% apraclonidine test in the diagnosis of oculosympathetic paresis (OSP).
Method: Apraclonidine (0.5%) was administered to 31 eyes, nine with a diagnosis of Horner syndrome (HS), 22 with bilateral OSP caused by diabetes, and to 54 control eyes. All were confirmed with the cocaine test. The effects on pupil diameter and upper eyelid level were observed 1 hour later.
Results: Apraclonidine caused a mean dilation of 2.04 mm (range 1–4.5) (p<0.001) in the pupils with OSP and it caused pupillary constriction in the control eyes with a mean change of -0.14 mm (range 0.5-1) (p<0.05). It caused reversal of anisocoria in all HS cases. Its effects on both pupil diameters and upper lid levels differed significantly between the groups (p<0.001). The mean elevation in the upper lid was 1.75 mm (range 1–4) in the OSP group (p<0.001) and 0.61 mm (range 0–3) in the control group (p<0.001).
Conclusion: The effect of the apraclonidine (0.5%) test on the pupil diameter was diagnostic for OSP and had at least the same sensitivity and specificity as the cocaine test for the diagnosis of OSP.
Adverse effects of apraclonidine used in the diagnosis of Horner syndrome in infants. Watts S, et al. Journal of AAPOS 2007:11(3):282-283
“The reported adverse effects included in this report suggest that apraclonidine should be used with caution or not at all, in infants under the age of 6 months. Though a reduced concentration of apraclonidine (0.5%) is also effective in the diagnosis of Horner syndrome,8 it is the immaturity of the blood-brain barrier in young infants that permits central nervous system depression suggesting that patients may still be at risk.9 If apraclonidine must be used in infants younger than 6 months of age, the patient should be observed for a period of at least 2 hours after instillation of the drops, with admission to a pediatric ward prompted by lethargy, bradycardia, or a reduced respiratory rate.”
Anisocoria Care Path
From: Clinical Pathways in Neuro-ophthalmology 2003
References:
1. Anisocoria in cats. http://www.lifelearn-cliented.com/cms/resources/body/6396/anisocoria-in-cats-1.jpg
2. Clinical Decisions in Neuro-Ophthalmology, Burde RM, Savino PJ & Trobe JD. 3nd Edition. Mosby 2002
3. Clinical Pathways in Neuro-ophthalmology:An Evidence-Based Approach. Lee AC & Brazis PW. Thieme 2003
4. Neuro-ophthalmology, Section 5, Basic and Clinical Science Course. American Academy of Ophthalmology 2013-2014
5. Anisocoria. Boisvert CJ,Tran N-A. EyeWiki. 2016. American Academy of Ophthalmology. http://eyewiki.aao.org/Anisocoria
More than 600 additional neuro-ophthalmology questions are freely available at http://EyeQuiz.com.
Questions prior to September 2016 are archived at http://ophthalmology.stanford.edu/blog/
After that, questions are archived at https://neuro-ophthalmology.stanford.edu
Follow https://twitter.com/NeuroOphthQandA to be notified of new neuro-ophthalmology questions of the week.
Please send feedback, questions and corrections to tcooper@stanford.edu.